GVP Audits
To guarantee adherence to industry standards and regulatory requirements, Pharmazone offers effective and efficient GVP auditing services. This promotes the safe and efficient use of pharmaceutical products. Pharmazone can offer knowledge and a setting that is always ready for an inspection thanks to its skilled and knowledgeable quality auditors who specialize in the PV and clinical domains.
GVP Audits
To guarantee adherence to industry standards and regulatory requirements, Pharmazone offers effective and efficient GVP auditing services. This promotes the safe and efficient use of pharmaceutical products. Pharmazone can offer knowledge and a setting that is always ready for an inspection thanks to its skilled and knowledgeable quality auditors who specialize in the PV and clinical domains.
Mandatory Audits for Regulatory Compliance
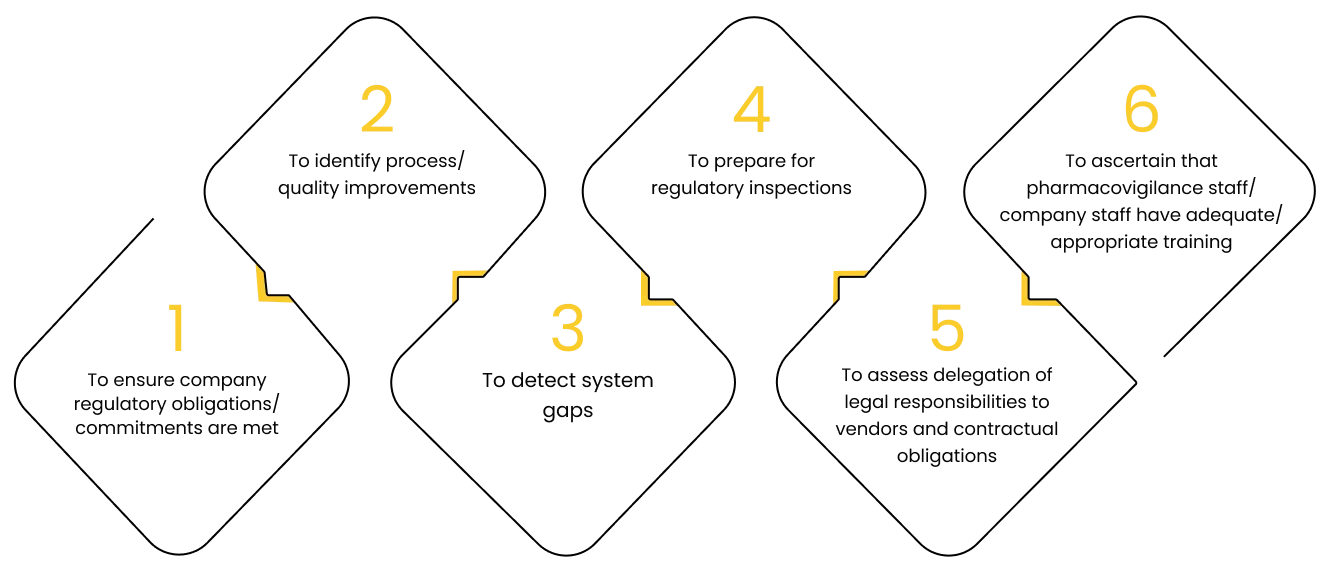
1
To ensure company regulatory obligations/ commitments are met
2
To identify process/ quality improvements
3
To detect system gaps
4
To prepare for regulatory inspections
5
To assess delegation of legal responsibilities to vendors and contractual obligations
6
To ascertain that pharmacovigilance staff/ company staff have adequate/ appropriate training

Audit Procedures

- Identify and confirm all roles/responsibilities of staff involved
- Obtain and review the documents requested by the auditors
- Prepare audit back-office
- Compile questions for relevant auditees
- Explain the audit and QA plan / scope of the audit
- Confirm date/time still ok
- Explain the course of the audit
- Request further documents that could not be requested earlier
- Interviews with the responsible staff on the processes/SOPs/workflows
- Document reviews
- Demonstration of activities
- Walk through the safety processing system (e.g., work area, file storage, archiving)
- Discussion of preliminary results
- Follow-up CAPAS
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification
A Pre-Inspection Gap Audit of a Contract Research Organization (CRO)/Site is a proactive assessment conducted before an official regulatory inspection to identify potential areas of non-compliance or gaps in quality management systems.
The primary purpose of a Pre-Inspection Gap Audit is to evaluate the CRO’s readiness for a regulatory inspection by identifying any deficiencies or gaps in compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and sponsor expectations. By conducting this audit proactively, the CRO can address any identified issues and strengthen its quality management systems before the official inspection.
Key Components Includes:
- Documentation Review
- Site Selection and Monitoring
- Vendor Management
- Process Evaluation
- Data Management and Quality Control
- Training and Personnel Qualification