GXP Audits
GXP Audits are an international ethical and scientific quality standard for the design, conduct, performance, monitoring, auditing, recording, analyses & reporting of clinical trials.
Quality
Quality and Compliance is a system for ensuring that products are consistently produced and controlled according to quality standards.
Choosing Pharmazone: A Closer Look
Our
Projects
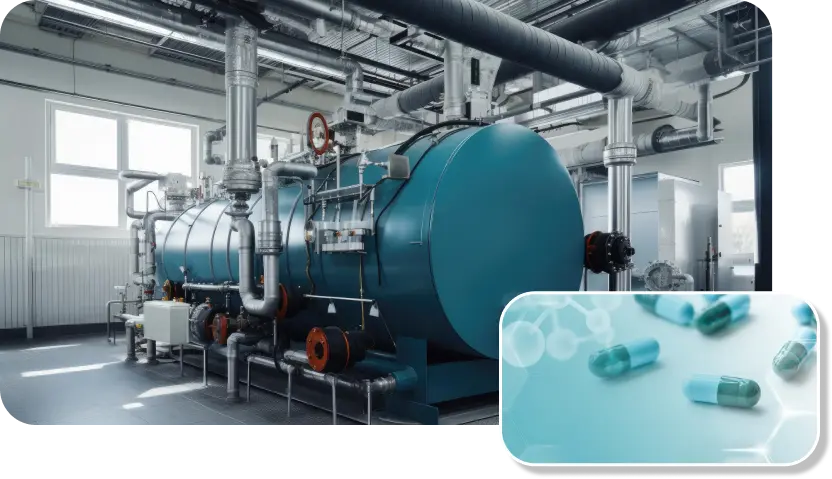
Plant Setup
Designed the state of art engineering setup of the Dry powder injection line manufacturing plant in North India. Validation batches are completed and now the Product manufacturing is started. WHO-GMP certificate is also received for the site. Now we are guiding the site for next objective of EUGMP product filing and EUGMP certification.

USFDA pre-inspection readiness
USFDA Pre inspection audit was conducted at site for the Inspection readiness. Experts involved in the thorough assessment of the all critical areas. The guidance and involvement of the Experts helped to clear the USFDA inspection without any observations.

FAT in China for the Vial line with Isolator
Planned and executed the critical FAT in China for the Vial line with isolators for leading pharmaceutical company in India. Our experts were involved in the thorough assessment and compliance for the equipment testing. It helped the client in receiving the equipment line as per the work order and with globally compliant documents.















